EPIDEMIOLOGY AND OPTIMAL PATIENT CARE
Steven Ferrucci, OD, FAAO, FORS
Retinal vein occlusions (RVOs) are the second most common type of retinal vascular disease, second only to diabetic retinopathy.1 The three types of RVOs are branch retinal vein occlusion (BRVO), central retinal vein occlusion (CRVO), and hemiretinal vein occlusion (HRVO).2 A BRVO is an occlusion of any branch or tributary of the central retinal vein, typically at an arteriovenous crossing.1,2 It presents clinically with pathological signs, such as hemorrhages, in a sectoral region of the retina.1,2 Oftentimes, the offending blood vessel can be identified by locating the area of pathology and tracing it back to the visibly occluded vessel.
A CRVO is an occlusion at or proximal to the location that the central retinal vein exits the eye.1,2 Unlike in BRVO, the site of vessel compression cannot be visualized because it occurs within the optic nerve head at the level of the lamina. Lastly, an HRVO is the least common type of RVO3 and occurs from an occlusion at the optic disc.2 Hemorrhages in an HRVO present clinically in the superior or inferior hemifield of the retina.2
Prevalence, Incidence, and Risk Factors
RVO incidence is estimated at 180,000 eyes per year in the United States, based on data from the US Census Bureau and the 2008 Beaver Dam Study.4-7 BRVOs, in particular, account for nearly 80% of RVOs.4-7 The mean age of onset of a BRVO is 65 years of age.7
Systemic risk factors for RVO include arteriosclerosis, hypertension, hyperlipidemia, and diabetes mellitus (specifically associated with CRVO).8 Ocular risk factors include ocular hypertension and primary open-angle glaucoma.1,9,10
The risk of RVO is greatest in patients with an RVO in the contralateral eye.11-13 Specifically, patients with unilateral BRVO have a 10% risk of developing bilateral RVO within 3 years,11 whereas patients with unilateral CRVO have a 7% risk of developing bilateral RVO after 5 years.12,13
Comprehensive Exam
Symptoms of RVO include painless blurring or loss of vision.10,14,15 Depending on the extent and location of the pathology, the vision loss in RVO can be quite variable, ranging from mild (VA of 20/20) to severe (VA of counting fingers).10,14,15 For example, a patient who has BRVO without macular involvement may have 20/20 VA. Indeed, if the macula is affected by edema or ischemia, then the visual acuity is typically reduced.
According to the American Academy of Ophthalmology Retinal Vein Occlusions Preferred Practice Pattern, the recommended clinical exam for RVO includes a traditional, comprehensive eye exam.2 Specifically, recommendations include measuring visual acuity, assessing pupils for a relative afferent pupillary defect, measuring IOP, and performing a slit lamp and dilated posterior segment exam.2 Of note, a relative afferent pupillary defect is more likely in CRVO than in BRVO.2
Late-stage complications of RVO include neovascularization of the iris (NVI) and/or angle (NVA); therefore, a careful slit lamp exam, including gonioscopy, is warranted in patients with RVO at presentation and follow-up visits.2 I recommend performing gonioscopy on a nondilated eye because it is easier to visualize NVI with a nondilated versus dilated eye. It is important to perform gonioscopy even in the absence of NVI since neovascularization may initially appear in the angle in about 10% of eyes.16 The role of imaging in RVO is discussed later in this educational activity.
Differential Diagnosis
The differential diagnosis of BRVO includes diabetic retinopathy, hypertensive retinopathy, radiation retinopathy, macular telangiectasia, and exudative perifoveal vascular anomalous complex.10,14,17,18 In CRVO, the differential diagnosis includes ocular ischemic syndrome, hyperviscosity retinopathy, and diabetic retinopathy.10,14,17,18 With a detailed history and eye exam, many differential diagnoses can be included or excluded. Keep in mind that many differential diagnoses of RVO present asymmetrically but bilaterally, whereas RVO is often unilateral.
Workup for Patients Older Than 50 Years
Workup recommendations for patients 50 years of age or older with RVO include evaluating for medical risk factors, primarily hypertension, diabetes, and hyperlipidemia.10,14,19-21 In an eye care office, the patient’s blood pressure should be evaluated same day. The patient should also be referred to a primary care physician to evaluate and potentially optimize blood pressure, blood glucose, and cholesterol.10,14,19-21 Additionally, the primary care physician should also be made aware of the higher risk of cardiovascular disease and stroke in patients presenting with RVO,2 so that a cardiovascular evaluation can be performed as the physician sees fit. I have found that although primary care physicians possess a wide range of knowledge, they may need a nudge in the right direction to perform appropriate testing for a patient who needs a workup for RVO.
Lastly, smoking cessation guidance is recommended because many studies have found that smoking is associated with RVO and other retinal diseases.10,14,19-21 For patients who smoke, give them simple guidance by explaining that smoking may contribute to certain eye diseases, such as RVO.
Workup for Patients Younger Than 50 Years
Workup recommendations for patients younger than 50 years of age are slightly more challenging. When patients present with RVO at a younger age, with bilateral RVO, with a family history of thrombosis, or a previous medical diagnosis of thrombosis, additional testing for inborn or acquired causes of hypercoagulability may be needed.10,14,19,20 It is helpful in these rare cases to refer the patient to a retina specialist who can coordinate with a hematologist.10,14,19,20
Hematologists may not understand the pathophysiology of RVO; therefore, eye care clinicians need to communicate on the possible underlying causes of the RVO. It is also important to differentiate hypercoagulable causes (in RVO) versus embolic causes (in retinal artery occlusions) because hematologists are oftentimes unfamiliar with these differing etiologies. Open collaboration across all health care specialties is important for optimal patient care.
Referral
It cannot be understated that a patient with treatable complications from RVO, such as macular edema or neovascularization of the anterior or posterior segment, requires a prompt referral to a retina specialist. Communication with a primary care physician is essential to evaluate for and to treat medical problems associated with RVO. Importantly, collaborate with the primary care physician and hematologist on appropriate testing to consider in the workup for patients with RVO, especially in younger individuals.
Q&A
Q: Carolyn E. Majcher, OD, FAAO, FORS: In addition to hypercoagulable causes, do you consider a workup for causes of retinal phlebitis, such as sarcoidosis, syphilis, or systemic lupus erythematosus?
A:Yasha S. Modi, MD, MHS: Great question. Sarcoidosis, syphilis, and systemic lupus erythematosus are rare causes of CRVO; however, they are included in the differential diagnosis of CRVO.22-24 A patient with either sarcoidosis or syphilis and an RVO presents with cells, or uveitis, in addition to signs of RVO.22,23 So, if an RVO presents with uveitis, then the workup should include an evaluation for sarcoidosis and syphilis.22,23 On the other hand, lupus typically causes an occlusive vasculitis that includes veins and arteries.24
1. Ip M, Hendrick A. Retinal vein occlusion review. Asia Pac J Ophthalmol (Phila). 2018;7(1):40-45.
2. Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal Vein Occlusions Preferred Practice Pattern® [published correction appears in Ophthalmology. 2020 Sep;127(9):1279]. Ophthalmology. 2020;127(2):P288-P320.
3. Harris A, Guidoboni G, Siesky B, et al. Ocular blood flow as a clinical observation: Value, limitations and data analysis [published online ahead of print, 2020 Jan 24]. Prog Retin Eye Res. 2020;100841.
4. Gewaily D, Greenberg PB. Intravitreal steroids versus observation for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2009;(1):CD007324. Published 2009 Jan 21.
5. US Census Bureau. Population and housing unit estimates. Accessed February 16, 2023. https://www.census.gov/programs-surveys/popest.html
6. Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(4):513-518.
7. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-1112.e1.
8. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126(5):692-699. doi:10.1001/archopht.126.5.692
9. Kolar P. Risk factors for central and branch retinal vein occlusion: A meta-analysis of published clinical data. J Ophthalmol. 2014;2014:724780.
10. Central retinal vein occlusion. EyeWiki. Nov 2022. Available at: https://eyewiki.aao.org/Central_Retinal_Vein_Occlusion#Risk_Factors. Accessed on February 16, 2023.
11. Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1094-1101.e5.
12. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117(4):429-441.
13. Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group [published correction appears in Arch Ophthalmol 1997 Oct;115(10):1275]. Arch Ophthalmol. 1997;115(4):486-491.
14. Branch retinal vein occlusion. EyeWiki. Nov 2022. Available at: https://eyewiki.org/Branch_Retinal_Vein_Occlusion#cite_ref-10. Accessed on February 14, 2023.
15. McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1113-1123.e15.
16. Central Vein Occlusion Study Group. Baseline and early natural history report. The Central Vein Occlusion Study. Arch Ophthalmol. 1993;11:1087-1095.
17. Blair K, Czyz CN. Central retinal vein occlusion. StatPearls [Internet]. Updated Oct 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK525985/. Accessed on February 16, 2023.
18. Cochran ML, Mahabadi N, Czyz CN. Branch retinal vein occlusion. StatPearls [Internet]. Updated Oct 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK535370/. Accessed on February 16, 2023.
19. Nicholson L, Talks SJ, Amoaku W, Talks K, Sivaprasad S. Retinal vein occlusion (RVO) guideline: executive summary. Eye (Lond). 2022;36(5):909-912.
20. Mahoney BP, Constantine V. Retinal and Retinal Vascular Emergencies. In: Nyman, J. Problems in Optometry; Ocular Emergencies. JB Lippincott Co, Philadelphia, PA 1989: 123-48.
21. Gurwood AS. A diagnosis in the same vein. Sept 2019. Available at https://www.reviewofoptometry.com/article/a-diagnosis-in-the-same-vein#footnotes. Accessed on February 14, 2023.
22. Shrestha R, Sitaula RK, Karki P, Joshi SN. Branch retinal vein occlusion in a case of sarcoidosis. Nepal J Ophthalmol. 2021;13(25):146-151.
23. Ophthalmologic manifestations of syphilis. EyeWiki. July 2022. Available at: https://eyewiki.aao.org/Ophthalmologic_Manifestations_of_Syphilis. Accessed September 5, 2023.
24. Kumar K, Dan S, Sinha TK, Bhattacharya D. Severe vaso-occlusive retinopathy in systemic lupus erythematosus: A case series. Cureus. 2021;13(1):e13019. Published 2021 Jan 30.
IDENTIFYING AND ASSESSING PATHOLOGY IN RVO WITH TODAY’S IMAGING MODALITIES
Carolyn E. Majcher, OD, FAAO, FORS
Acute Versus Chronic RVO
Pathological findings in retinal venous occlusive disease are often unilateral and asymmetric between the eyes.1 In branch retinal vein occlusion (BRVO) specifically, the pathology is typically localized to one quadrant or sector of the fundus.2 Retinal vein occlusions (RVOs) can be differentiated into acute or chronic, with each having unique clinical signs. The pathologic findings in acute central retinal vein occlusion (CRVO) are typically present in all four quadrants of the fundus and include characteristic dilated and tortuous veins, intraretinal telangiectasia, and intraretinal hemorrhaging.3 Other acute signs include cotton wool spots, disc edema, and macular edema.3
In late or chronic stages of RVO, collateral vessels may develop to shunt blood around the occlusion or the thrombosis.2-5 These vessels may arise at the optic disc in CRVO or in the area between the perfused and nonperfused retina (often crossing the horizontal raphe) in temporal BRVO.2-5 Collateral vessels form within the retina and can therefore be differentiated from neovascularization, which grows anterior to the retina, using depth-resolved OCT and OCT angiography (OCTA).
Several other chronic signs may be present, such as vessel sclerosis/attenuation, telangiectatic vessels, and neovascularization of the optic disc and retina, which can be complicated by preretinal/vitreous hemorrhage and localized tractional retinal detachment.2-5 Anterior segment neovascularization of the iris (NVI) and/or angle is a potential consequence of ischemic CRVO that may result in painful and blinding neovascular glaucoma.2-5 Lastly, retinal macular exudate is another common feature of chronic RVO, and macular edema may persist late.6 Of note, the degree of exudate may increase after successful anti-VEGF therapy.
Ischemic Versus Nonischemic RVO
In RVO, it is critical to use exam findings to clinically distinguish whether the CRVO is ischemic or nonischemic, given the prognostic factors of each subtype. An ischemic CRVO has about a 50% to 60% incidence of anterior segment neovascularization, which typically develops within 3 to 5 months.7 Therefore, an ischemic CRVO is considered high risk and requires close monitoring and referral to a retina specialist.7
Exam findings suggestive of an ischemic CRVO include retinal capillary nonperfusion equal to or greater than 10 disc areas on standard fluorescein angiography (FA), VA worse than 20/200, a relative afferent pupillary defect, severe hemorrhaging and cotton wool spots (commonly referred to as “blood and thunder” appearance), and significant visual field defects.3,4,7
When neovascularization of the anterior segment develops in ischemic RVO, it may present as NVI or neovascularization of the angle (NVA).3 Prior to dilation, eye care providers should vigilantly screen for anterior segment neovascularization, including magnified iris examination and gonioscopy. NVA is best visualized by performing gonioscopy and is seen as fine, vertically oriented, red vessels that cross the scleral spur and then proliferate horizontally along the pigmented trabecular meshwork, giving it a reddish hue. Over time, peripheral anterior synechiae may form in the angle, leading to potentially blinding and painful neovascular glaucoma.3,4
Importantly, patients with NVI or NVA require a prompt referral to a retina specialist for acute treatment. Fast-acting anti-VEGF therapy, followed by panretinal photocoagulation, is the current standard of care to quickly regress anterior segment neovascularization before peripheral anterior synechiae forms. Early detection and prompt referral of neovascularization is critical to optimize outcomes and may spare patients from needing invasive glaucoma surgeries.
Imaging Modalities
Multimodal retinal imaging in the assessment of RVO includes color fundus photography (CFP), OCT, OCTA, and FA. Widefield and ultra-widefield CFP aids in detecting, documenting, and monitoring peripheral retinal hemorrhages. It may also highlight subtle, localized pathology, such as asymmetry in the retinal vasculature. For example, localized dot-blot hemorrhages and dilated, tortuous veins may be visible on CFP in the inferior half of the fundus, which are classic characteristics of a hemiretinal vein occlusion (HRVO). Vascular sheathing, which indicates significant retinal nonperfusion and is seen as whitening of the retinal vessels, may also be visible on widefield and ultra-widefield CFP in late-stage ischemic CRVO.
OCT has incredible clinical value by detecting macular edema, which may complicate both CRVO and BRVO. In addition, OCT can be used to quantitatively track resolution or worsening of macular edema that guides anti-VEGF therapy. As a case example, I had a patient who presented with classic features of CRVO in one eye that was apparent on CFP. This patient had intraretinal hemorrhaging and dilated tortuous veins in all four quadrants of his fundus. Interestingly, they also had retinal characteristics consistent with a central retinal artery occlusion, including pallid, macular swelling and a foveal “cherry red spot.” OCT imaging for this patient showed classic signs of macular edema, in addition to hyperreflective inner retinal thickening from infarction that accompanies central retinal artery occlusions. So, I worked up the patient for thrombolic and embolic systemic etiologies.
Remember that vision loss in RVO can be a consequence of not only macular edema but also macular ischemia, which may be identified on OCTA. I had a patient present with count fingers VA and a CRVO. Although anti-VEGF therapy resolved most of her macular edema, her BCVA improved minimally to 20/250 (Figure, lower left images). OCTA unfortunately revealed macular ischemia worse in the deep capillary plexus and within the inferior macula, visualized as enlargement of the foveal avascular zone and retinal nonperfusion (Figure, red box).
 Figure. OCTA imaging of a patient with macular ischemia due to CRVO before and after anti-VEGF therapy. Image courtesy of Carolyn E. Majcher, OD, FAAO, FORS.
Figure. OCTA imaging of a patient with macular ischemia due to CRVO before and after anti-VEGF therapy. Image courtesy of Carolyn E. Majcher, OD, FAAO, FORS.Even with successful anti-VEGF treatment, macular ischemia is, unfortunately, irreversible and limits the visual prognosis. OCTA can identify retinal nonperfusion, which is nearly invisible on clinical examination alone. It is valuable in estimating the degree of retinal nonperfusion and in identifying an ischemic versus a nonischemic RVO (to reiterate, this distinction predicts the risk for associated anterior or posterior segment neovascularization).
Since OCTA provides a volumetric dataset, blood flow anterior to the retina may be isolated out on imaging to view preretinal neovascularization and to easily identify neovascularization of the optic disc or elsewhere. When examining OCTA imaging, it is important to cross-section through the area of potential neovascularization on the vitreoretinal interface to confirm that perfused tissue on top of the retina is present and to rule out segmentation error.
OCTA may also highlight RVOs that are subtle on clinical examination. I had an 83-year-old patient with Type 2 diabetes who presented for a primary open-angle glaucoma follow-up. I observed some abnormal vessels in the peripapillary disc region in the left eye, suspicious for either neovascularization of the disc from proliferative diabetic retinopathy or collateral vessels from a chronic RVO. OCTA imaging on this patient revealed retinal telangiectasia and nonperfusion localized in the superior nasal sector of the fundus, which confirmed diagnosis of a classic RVO.
Lastly, ultra-widefield FA holds substantial clinical value in RVO by highlighting the full extent of capillary nonperfusion. It can cover an angle of 200°, or about 80% of the retinal surface. Research has shown that the extent of retinal nonperfusion has prognostic value by appearing to correlate with the risk of future neovascularization growth and with the final visual acuity.8,9
Specifically, one study assessed the correlation of the ischemic index (defined as the ratio of nonperfused retinal area to total visible fundus area) with anterior or posterior segment neovascularization in CRVO.8 Fifteen eyes with neovascularization had a mean ischemic index of 75%, whereas the eyes without neovascularization had a mean ischemic index of only 6%.8 Notably, all eyes with neovascularization had an ischemic index greater than 45%.8
Another study examined the correlation between the extent of retinal nonperfusion and the final visual acuity in BRVO.9 Eyes with 50- to 100-disc diameters in retinal nonperfusion had a final BCVA of 35 letters, whereas those with less than 50- to 100-disc diameters in retinal nonperfusion had a BCVA of 40.6 letters.9
1. Varner P. Significance of asymmetric retinal vasculature tortuosity- report of 10 consecutive cases. Acta Scientific Ophthalmology. 2019;2.1:02-06. Accessed August 8, 2023. Available at: https://actascientific.com/ASOP/pdf/ASOP-01-0020.pdf
2. Branch retinal vein occlusion. EyeWiki. Nov 2022. Available at: https://eyewiki.org/Branch_Retinal_Vein_Occlusion#cite_ref-10. Accessed on February 14, 2023.
3. Central retinal vein occlusion. EyeWiki. Nov 2022. Available at: https://eyewiki.aao.org/Central_Retinal_Vein_Occlusion#Risk_Factors. Accessed on February 16, 2023.
4. Blair K, Czyz CN. Central retinal vein occlusion. StatPearls [Internet]. Updated Oct 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK525985/. Accessed on February 16, 2023.
5. Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33(5):901-910.
6. Hayreh SS, Zimmerman MB. Fundus changes in central retinal vein occlusion. Retina. 2015;35(1):29-42.
7. Glaucoma, excerpt. In: Tanna AP, Boland MV, Giaconi JA, et al, eds. Basic and Clinical Science Course. 2020-2021. AAO; part II, chapter 06. Accessed August 8, 2023. https://www.aao.org/education/bcscsnippetdetail.aspx?id=e857ff6d-0763-419c-a22a-1aaa50605a6b
8. Tsui I, Kaines A, Havunjian MA, et al. Ischemic index and neovascularization in central retinal vein occlusion. Retina. 2011;31(1):105-110.
9. Abri Aghdam K, Reznicek L, Soltan Sanjari M, et al. Peripheral retinal non-perfusion and treatment response in branch retinal vein occlusion. Int J Ophthalmol. 2016;9(6):858-862. Published 2016 Jun 18.
TREATMENTS FOR RVO
Yasha S. Modi, MD, MHS
Evidence for Treating RVO Early to Restore Vision
Treatment for RVO (retinal vein occlusion) is aimed at treating the sequelae of the occlusion, not the occlusion itself.1 Cystoid macular edema is the most common vision-threatening complication of RVO.2 Three therapies have been mostly used by retina specialists to treat macular edema in RVO: (1) Anti-VEGF therapy (ie, ranibizumab, aflibercept, and off-label bevacizumab) is recommended as first-line treatment for RVO because of its proven efficacy and safety, and it has become the gold standard1; (2) Steroid therapies, namely intravitreal triamcinolone injection and dexamethasone implant, may be useful in patients who are recalcitrant to anti-VEGF therapy1; (3) Laser photocoagulation is indicated for neovascular complications in the areas of peripheral nonperfusion.1 Although lasers are rarely used today, focal laser is indicated for branch retinal vein occlusion (BRVO), whereas macular laser is indicated for central retinal vein occlusion (CRVO).1
Many pivotal clinical trials provide Level 1 evidence for anti-VEGF, steroid, and laser photocoagulation therapy in RVO.3-17 Even though most of these trials were published over a decade ago, they continue to hold important lessons for clinical care today.
BRAVO and CRUISE
Regarding anti-VEGF therapy, the BRAVO and CRUISE studies evaluated the efficacy and safety of ranibizumab in treating macular edema following BRVO and CRVO, respectively.18,19 As an aside, use of sham treatments that did not contain a drug was considered ethically appropriate because no FDA-approved therapy existed for RVO at the time of these clinical trials. BRAVO and CRUISE similarly showed that the BCVA in patients with macular edema from RVO receiving 0.3 mg or 0.5 mg ranibizumab intravitreal injections compared with sham injections improved significantly in the mean change from baseline BCVA letter score during the first three injections, and started to plateau by the fourth or fifth injection.18,19 In the BRAVO study, the mean change at month 6 from baseline BCVA letter score was greater in the ranibizumab group (16.6, 0.3 mg; 18.3, 0.5 mg; P < .0001) than in the sham group (7.3).18 In the CRUISE study, the mean change at month 6 from baseline BCVA letter score was also greater in the ranibizumab group (12.7, 0.3 mg; 14.9, 0.5 mg; P < .0001) than in the sham group (0.8).19
In the BRAVO and CRUISE studies, patients in the sham group were crossed over to treatment with ranibizumab at 6 months.18,19 In the BRAVO study, the mean change from baseline BCVA letter score in the sham group (12.1) was significantly less than in the ranibizumab group (16.4, 0.3 mg; 18.3, 0.5 mg; P < .05) at month 12.18 Similarly, in the CRUISE study, the mean change from baseline BCVA letter score in the sham group (7.3) was significantly less than in the ranibizumab group (13.9, 0.3 mg; 13.9, 0.5 mg; P < .05) at month 12.19 Therefore, patients who received delayed treatment of anti-VEGF therapy by 6 months had worse visual outcomes than patients who received immediate treatment with ranibizumab.18,19 The message here is that it may be incumbent on the physician to encourage timely treatment to avoid leaving vision “on the table” for patients who are “on the fence” regarding withholding treatment.
The BRAVO and CRUISE studies also found that anti-VEGF therapy in patients with RVO decreases the amount of macular fluid or the central foveal thickness (CFT) quickly.18,19 The earliest timepoint that showed a significant difference in CFT between the 0.3 mg and 0.5 mg ranibizumab groups and the sham group was at day 7.18,19 Additionally, the BRAVO and CRUISE studies found that the reduction in CFT stabilized after its initial improvement in the ranibizumab groups with PRN treatment.18,19 At month 12, the mean reduction from baseline CFT in the 0.3 mg and 0.5 mg ranibizumab groups was 313.6 mm and 347.4 mm, respectively, in the BRAVO study18 and 452.8 mm and 462.1 mm, respectively, in the CRUISE study.19
The findings from these 2011 studies also highlight today’s expectations and outcomes in treating patients who experience RVO with anti-VEGF therapy. Notably, the main difference in modern treatment compared to treatment in the original studies is the frequency of dosing anti-VEGF intravitreal injections. Today, most US retina specialists employ a treat-and-extend strategy, which increases the dosing interval based on evaluating the retinal anatomy, whereas the original studies followed a regular monthly dosing strategy.18,19 Treat-and-extend dosing may be thought of as a marriage of PRN dosing (or injecting with recurrence of macular edema only) and monthly therapy, which is a highly frequent injection strategy.
CENTERA
The 2021 CENTERA (Evaluation of a Treat and Extend Regimen of Intravitreal Aflibercept for Macular Edema Secondary to CRVO; NCT02800642) study was an open-label, phase 4 study that assessed the efficacy and safety of aflibercept intravitreal injections dosed with a treat-and-extend dosing regimen in patients with macular edema from a CRVO.20 Patients were treated with 2 mg of intravitreal aflibercept injections at baseline followed by every 4 weeks until the disease stabilized or until week 20, then the treatment dosing interval was adjusted according to functional and anatomic outcomes.20
This study showed that the treat-and-extend dosing regimen of aflibercept significantly improved the mean change in BCVA from baseline (51.9 letters) to week 76 (72.3 letters) in patients with macular edema from CRVO (Figure 1).20 In addition, 72 (45%) patients receiving aflibercept achieved a mean treatment interval of 8 or more weeks in the treat-and-extend phase.20 The mean dosing interval in the treat-and-extend phase for patients receiving intravitreal aflibercept who completed treatment was 7.6 + 1.9 weeks,20 which is considerably longer than a monthly dosing regimen.
The study also showed that central retinal thickness (CRT) was reduced quickly from 759.9 mm at baseline to 300.4 mm at 4 weeks (mean change: -461.8 mm) and stabilized with the treat-and-extend dosing regimen of intravitreal aflibercept in patients with CRVO (Figure 2).20
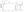 Figure 1. Mean change in BCVA from baseline to week 76 in patients receiving intravitreal aflibercept.20
Figure 1. Mean change in BCVA from baseline to week 76 in patients receiving intravitreal aflibercept.20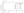 Figure 2. Mean change in CRT from baseline to week 76 in patients receiving intravitreal aflibercept.20
Figure 2. Mean change in CRT from baseline to week 76 in patients receiving intravitreal aflibercept.20Comparative Studies Among anti-VEGF Therapies
Among anti-VEGF therapies, is one therapy more clinically efficacious in treating RVO than the others? The Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) aimed to answer this question by comparing the efficacy of intravitreal anti-VEGF therapies in eyes with macular edema secondary to RVO.21
SCORE2
SCORE2 was a multicenter, randomized clinical trial that compared the efficacy of aflibercept to off-label bevacizumab for the treatment of macular edema due to CRVO or hemiretinal vein occlusion (HRVO).21 Patients (n=362) were randomized 1:1 to receive intravitreal injections of bevacizumab (1.25 mg) or aflibercept (2.0 mg) every 4 weeks for 6 months.21 The study showed that off-label bevacizumab was noninferior to aflibercept with respect to mean change in visual acuity letter score for 6 months (18.6 bevacizumab vs 18.9 aflibercept; 97.5% CI, -3.1 to ∞; with noninferiority margin of -5.0 letters).21 This population of patients overall had similar visual acuity outcomes with either aflibercept or off-label bevacizumab.21 However, remember that the visual outcome seen in SCORE2 is not what we expect clinically for every patient treated with anti-VEGF therapy for macular edema due to CRVO.
The SCORE2 study also found that 91% of patients who received aflibercept and 78% of patients who received off-label bevacizumab were considered good responders, as defined by a protocol that included visual acuity and OCT.21 The researchers wondered whether the injection frequency was too high and if some patients should be switched from monthly to treat-and-extend dosing. As a result, patients who had a good response to treatment at month 6 were rerandomized to continue either monthly or treat-and-extend dosing.21 At month 12, patients treated with either monthly or treat-and-extend dosing of anti-VEGF therapy showed no difference in visual acuity or in central subfield thickness (CST).21
Keep in mind that the visual acuity of participants had great recovery most likely because the investigators followed strict protocols in the first 12 months of SCORE2.21 In contrast, patient follow-up between months 12 and 24 was at the discretion of the investigators.22 The study found that mean visual acuity decreased by 5 letters in the patients from month 12 to 24, with no difference in visual acuity based on the original drug allocated.22 However, the mean visual acuity compared to the baseline visual acuity was overall markedly improved by 15 letters (3 lines).22 The take-away message here is that clinical trials employ strict follow-up protocols with participants, whereas clinicians may use less stringent follow-up protocols with real-world patients.
Visual Significance of Macular Ischemia
We know that patients with RVO may lose vision from two complications—macular edema and macular ischemia—and that anti-VEGF therapy treats macular edema, not ischemia. A 2022 study by Spooner et al showed the limitations of treatment with anti-VEGF therapy.23 It determined that within year 1 of treatment patients who are good, moderate, or poor responders to anti-VEGF therapy stratify into a final visual acuity (Figure 3),23 which may be referred to as the “swim lane” or ceiling effect. Although there is a lack of clear understanding of the underlying mechanism for this “swim lane” or ceiling effect, it is thought that recalcitrant macular edema and severe macular ischemia ultimately limit the improvement in visual acuity.23 So, while anti-VEGF therapy is quite beneficial, oftentimes the visual outcomes may be preordained at the initial disease presentation.
 Figure 3. A limitation of treatment with anti-VEGF therapy is that the final visual acuity is stratified by the initial visual acuity.23
Figure 3. A limitation of treatment with anti-VEGF therapy is that the final visual acuity is stratified by the initial visual acuity.23Effect of Treatment Frequency on Real-World Outcomes
We have seen the effect of various dosing strategies of anti-VEGF intravitreal injections on outcomes in patients participating in clinical trials. However, does injection frequency matter in real-world patients? A 2021 retrospective analysis assessed data from the electronic medical records of patients from the Vestrum Health Treatment and Outcomes database of 251 retina specialists.24,25 The year 1 cohort in this analysis included 2,458 eyes,24,25 which is a considerably greater number than in most randomized clinical trials. The value of using the Vestrum database is in the large amount of data and in the relatively granular, proposed questions. The disadvantage of large datasets can be the “noise,” but the increase in numbers offsets this disadvantage.
The overall goal of this 2021 analysis was to determine whether patients have poorer outcomes in the real world because of less frequency in injections or follow-ups.24,25 This study analyzed treatment-naïve eyes with macular edema due to CRVO receiving anti-VEGF therapy between 2012 and 2016 with at least 1 year of follow-up.24,25 Researchers divided eyes into the following two dosing subgroups: (1) eyes that received six injections or fewer and those that received seven injections or more for up to 2 years; and (2) eyes that received one to three, four to six, seven to nine, and 10 injections or more for 1 year. Visual acuity was reported in visual acuity score (VAS) at baseline and quarterly subsequently.24,25
The study showed that the mean letter gain in VAS from baseline in eyes receiving seven injections or more (n=1607; 65%) was significantly greater than those receiving six injections or fewer (n=851; 35%) at year 1 (12.2 letters vs 7.0 letters; P < .001).24,25 The eyes that were stratified further, ie, one to three, four to six, seven to nine, and 10 injections or more for 1 year, had the “swim lane” or ceiling effect in improved mean VAS score (Figure 4).24,25 In addition, eyes receiving seven to nine injections and 10 injections or more compared to six injections or fewer resulted in a “plateau phenomenon” in improved mean VAS score but a better mean VAS score from baseline through year 1 (Figure 4).24,25 Not surprisingly, eyes that received six injections or fewer compared to those that received seven injections or more had greater variation in visual acuity and CFT through year 2.24,25
 Figure 4. The mean VAS change through year 1 by injection frequency in eyes with macular edema due to CRVO.24,25
Figure 4. The mean VAS change through year 1 by injection frequency in eyes with macular edema due to CRVO.24,25Dual-Pathway Therapy on the Horizon
A new drug for treatment of RVO is in the pipeline for patients who are poor responders to other anti-VEGF therapies. Faricimab blocks two disease pathways, VEGF-A and angiopoietin-2 (ang-2), and in 2022 gained FDA approval for the treatment of neovascular age-related macular degeneration and diabetic macular edema.26 In early 2023, the two phase 3 clinical trials BALATON and COMINO evaluated the efficacy and safety of faricimab compared to aflibercept in macular edema secondary to RVO.27 BALATON and COMINO included patients with BRVO (n=553) and either HRVO or CRVO (n=729), respectively.27 Patients were randomized 1:1 and treated with six monthly injections of either 6.0 mg of faricimab or 2.0 mg of aflibercept for 20 weeks.27
The studies showed that faricimab achieved noninferiority to aflibercept in visual acuity gains and loss of 15 or more BCVA letters at week 24.27 In the BALATON study, specifically, the percentage of patients who achieved a BCVA gain of 15 or more letters was comparable across treatment arms (56.1% faricimab vs 60.4% aflibercept; Figure 5).27 In the COMINO study, the percentage of patients who achieved a BCVA gain of 15 or more letters was also comparable across treatment arms (56.6% faricimab vs 58.1% aflibercept; Figure 5).27
 Figure 5. A comparable percentage of patients either gaining or losing vision with faricimab versus aflibercept at week 24.27
Figure 5. A comparable percentage of patients either gaining or losing vision with faricimab versus aflibercept at week 24.27 In both studies the faricimab treatment arm also achieved robust reductions in CST that were comparable to the aflibercept arm.27 In particular, in the BALATON study, the CST reductions were -311.4 mm and -304.4 mm in patients receiving faricimab and aflibercept, respectively.27 In the COMINO study, the CST reductions were -461.6 mm and -448.8 mm in patients receiving faricimab and aflibercept, respectively.27 The safety profile of faricimab was deemed consistent with previous trials, and all study arms had similar safety results.27
Interestingly, the BALATON and COMINO studies also evaluated FA imaging of the faricimab and aflibercept treatment arms to determine the proportion of patients with absence of macular leakage on FA at week 24.27 These studies showed that the proportion of patients with absence of macular leakage was greater in patients receiving faricimab (33.6% in BALATON; 44.4% in COMINO) than in those receiving aflibercept (21.0% in BALATON; 30.0% in COMINO).27 I think that this finding may stem from faricimab’s uniqueness in blocking both VEGF-A and ang-2, a growth factor believed to destabilize endothelial cells potentially leading to macular leakage.26
1. Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal Vein Occlusions Preferred Practice Pattern® [published correction appears in Ophthalmology. 2020 Sep;127(9):1279]. Ophthalmology. 2020;127(2):P288-P320.
2. Ip M, Hendrick A. Retinal vein occlusion review. Asia Pac J Ophthalmol (Phila). 2018;7(1):40-45.
3. Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: Six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1102-1112.e1.
4. Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984;98(3):271-282.
5. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102(10):1434-1444.
6. Tadayoni R, Waldstein SM, Boscia F, et al. Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion: 24-month results of the BRIGHTER Study [published correction appears in Ophthalmology. 2018 Mar;125(3):463]. Ophthalmology. 2017;124(12):1778-1787.
7. Campochiaro PA, Sophie R, Pearlman J, et al. Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology. 2014;121(1):209-219.
8. Scott IU, Ip MS, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6 [published correction appears in Arch Ophthalmol. 2009 Dec;127(12):1655]. Arch Ophthalmol. 2009;127(9):1115-1128.
9. Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134-1146.e3.
10. Haller JA, Bandello F, Belfort R Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12):2453-2460.
11. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117(6):1124-1133.e1.
12. Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119(4):802-809.
13. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155(3):429-437.e7.
14. Heier JS, Clark WL, Boyer DS, et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: two-year results from the COPERNICUS study [published correction appears in Ophthalmology. 2014 Nov;121(11):2293]. Ophthalmology. 2014;121(7):1414-1420.e1.
15. Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: One-year results of the phase 3 GALILEO study. Ophthalmology. 2014;121(1):202-208.
16. Campochiaro PA, Clark WL, Boyer DS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: The 24-week results of the VIBRANT study. Ophthalmology. 2015;122(3):538-544.
17. Clark WL, Boyer DS, Heier JS, et al. Intravitreal Aflibercept for Macular Edema Following Branch Retinal Vein Occlusion: 52-Week Results of the VIBRANT Study. Ophthalmology. 2016;123(2):330-336.
18. Brown DM, Campochiaro PA, Bhisitkul RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594-1602.
19. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041-2049.
20. Korobelnik JF, Larsen M, Eter N, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend for macular edema in central retinal vein occlusion: The CENTERA Study. Am J Ophthalmol. 2021;227:106-115.
21. Scott IU, VanVeldhuisen PC, Ip MS, et al. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion: The SCORE2 randomized clinical trial. JAMA. 2017;317(20):2072-2087.
22. Scott IU, Oden NL, VanVeldhuisen PC, et al. Month 24 outcomes after treatment initiation with anti-vascular endothelial growth factor therapy for macular edema due to central retinal or hemiretinal vein occlusion: SCORE2 Report 10: A secondary analysis of the SCORE2 randomized clinical trial. JAMA Ophthalmol. 2019;137(12):1389-1398.
23. Spooner KL, Fraser-Bell S, Hong T, Wong JG, Chang AA. Long-term outcomes of anti-VEGF treatment of retinal vein occlusion. Eye (Lond). 2022;36(6):1194-1201.
24. Modi YS, et al. Outcomes of intravitreal anti-vascular endothelial growth factor therapy for retinal vein occlusion in routine clinical practice. Presented at: Retina World Congress; March 21-24, 2019; Fort Lauderdale, FL.
25. Modi YS, Goduni L, Moini H, et al. Antivascular endothelial growth factor dosing frequency and visual outcomes in macular edema following central retinal vein occlusion. J Vitreoretin Dis. 2021;5(6):505-512.
26. Vabysmo. Prescribing information. Genentech Inc; 2023.
27. Genentech. New phase III data show Genentech’s Vabysmo rapidly improved vision and reduced retinal fluid in people with retinal vein occlusion (RVO). Available at: https://www.gene.com/media/press-releases/14983/2023-02-09/new-phase-iii-data-show-genentechs-vabys. Accessed on February 16, 2023.
CASE 1: RISK FACTORS OF RVO
Steven Ferrucci, OD, FAAO, FORS
Dr. Ferrucci: A 73-year-old man presented to the clinic with decreased vision in his left eye for 2 weeks. He had a history of type 2 diabetes without retinopathy for 11 years. His last HbA1c was 8.7%. He also had a history of systemic hypertension. BCVA was 20/50 in his left eye. His fundus examination revealed sectoral dot/blot and flame-shaped hemorrhages in the superior temporal quadrant. Macular edema was also evident on the clinical exam and confirmed with an OCT scan, indicated by a central retinal thickness of 470 mm (Figure 1). Given the patient’s risk factors for retinal vein occlusion (RVO), including hypertension, I checked the patient’s blood pressure in office. His blood pressure was normal at 125/82. I diagnosed this patient with a branch retinal vein occlusion (BRVO).
I referred this patient to his primary care physician to optimize his diabetes and other risk factors. I also referred him to the retina specialist for possible anti-VEGF therapy within 2 weeks. What do you think about this referral timeframe?
 Figure 1. OCT imaging in a patient with decreased vision. Image courtesy of Steven Ferrucci, OD, FAAO.
Figure 1. OCT imaging in a patient with decreased vision. Image courtesy of Steven Ferrucci, OD, FAAO.Yasha S. Modi, MD, MHS: I think that 1 to 2 weeks is a perfectly reasonable timeframe for this patient.
Dr. Ferrucci: I like to preappoint the patient at 3 months to “close the loop” on follow-up. This way I can ask the patient, “Did you see the retina specialist? What is the plan?” I can certainly also address any other issues that may have arisen.
Carolyn E. Majcher, OD, FAAO, FORS: I like to perform OCT at the follow-up to confirm the central macula is clear of substantial fluid before I prescribe a spectacle prescription. If the patient has significantly reduced vision from the RVO, full-time wear of trivex or polycarbonate lenses should be prescribed for protection.
Dr. Ferrucci: The retina specialist agreed with the diagnosis of BRVO with macular edema, and recommended serial anti-VEGF injections. The patient’s primary care physician altered his diabetic medications and recommended a telenutrition appointment for tighter glycemic control. Six months later, the patient’s HbA1c improved to 6.3%, his macular edema had resolved with about three injections, and his BCVA was 20/20. The patient continued to follow-up with the retina specialist, and I scheduled a 6-month follow-up.
Dr. Modi: This case demonstrates that if we communicate to patients that their eye issue is a fundamental problem of poor control of their blood pressure, blood sugar, and/or cholesterol, then that provides an impetus for the patient to try and improve their systemic health.
CASE 2: RVO VERSUS AMD
Carolyn E. Majcher, OD, FAAO, FORS
Dr. Majcher: A 70-year-old woman presented to the clinic with decreased vision and blur in her left eye for about 3 months. She had a systemic history of hypertension (in office blood pressure was 131/83), type 2 diabetes (last HbA1c was 6.5%), sleep apnea, chronic kidney disease, and hyperlipidemia. Her BCVA was 20/150 in the left eye. Color fundus photography (CFP) showed a large macular hemorrhage that appeared mostly striated and, therefore, in the superficial nerve fiber layer. There also appeared to be deeper, subretinal hemorrhaging and subretinal fluid.
Her retinal vasculature overall showed arteriovenous nicking and a reduced arteriovenous ratio bilaterally, both findings consistent with hypertensive retinopathy. The OCT scan of the macula revealed superficial hyperreflective retinal thickening with posterior shadowing, consistent with an intraretinal hemorrhage (Figure 2). Other macular scans showed intraretinal cystic fluid and substantial subretinal fluid (Figure 2). Hyperreflective material was apparent within the subretinal fluid, likely representing subretinal hemorrhage or proteinaceous exudate (Figure 2).
 Figure 2. CFP and OCT imaging in a patient with blur in the left eye. Image courtesy of Carolyn E. Majcher, OD, FAAO, FORS.
Figure 2. CFP and OCT imaging in a patient with blur in the left eye. Image courtesy of Carolyn E. Majcher, OD, FAAO, FORS.Yasha S. Modi, MD, MHS: Because of blockage of a blood vessel, blood and fluid can effectively traverse into all the retinal layers over time. To point out the chronicity of the RVO in this patient, look at the second and third OCT images that show the blood breaking into the outer plexiform layer and then traversing into the subretinal space (Figure 2). Subretinal hyperreflective material yields a short differential diagnosis, which includes blood, fibrin, proteinaceous exudates, or scarring. This case emphasizes the use of imaging to answer the diagnostic dilemma.
Dr. Majcher: The 6mm OCT angiography (OCTA) imaging was critical in clinching the diagnosis of RVO and ruling out exudative, neovascular AMD. The superior nasal, parafoveal region on the superficial capillary plexus preset revealed dilated, telangiectatic capillaries, which are consistent with RVO. Meanwhile, analysis of the outer retina choriocapillaris preset confirmed the absence of choroidal neovascularization.. We diagnosed her with a “twig” or macular RVO with severe macular edema.
Our plan was to refer this patient to a retina specialist for consideration of anti-VEGF therapy. We communicated with her primary care physician on the importance of controlling systemic vascular risk factors, including hypertension, diabetes, cholesterol levels, and cessation of smoking, to reduce the risk of a fellow eye RVO. We also emphasized that the patient had a higher risk of cardiovascular disease and stroke secondary to RVO.1
When considering AMD as a differential diagnosis, look for pigment epithelial detachment, drusen in the affected or fellow eye, and a deeper, subretinal or subpigment epithelium location of the hemorrhage, all suggestive of AMD (Figure 3, red box). OCTA can clinch the diagnosis by allowing visualization of a choroidal neovascular membrane in AMD.
Remember that AMD causes a breakdown of the outer blood-retinal barrier, so choroidal neovascularization appears in the outer retina choriocapillaris on OCTA. In contrast, RVO causes a breakdown of the inner blood-retinal barrier, so abnormalities present as nonperfusion and capillary telangiectasia in the superficial and deep capillary plexi (Figure 3, yellow box).
 Figure 3. OCT, CFP, and OCTA imaging in a patient with RVO compared to a patient with AMD. Image Key: Yellow Arrow = Abnormal Inner Retinal Vessels; Red Arrow = Abnormal Outer Retinal Vessels, Choroidal Neovascular Membrane. Image courtesy of Carolyn E. Majcher, OD, FAAO, FORS.
Figure 3. OCT, CFP, and OCTA imaging in a patient with RVO compared to a patient with AMD. Image Key: Yellow Arrow = Abnormal Inner Retinal Vessels; Red Arrow = Abnormal Outer Retinal Vessels, Choroidal Neovascular Membrane. Image courtesy of Carolyn E. Majcher, OD, FAAO, FORS.1. Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal Vein Occlusions Preferred Practice Pattern® [published correction appears in Ophthalmology. 2020 Sep;127(9):1279]. Ophthalmology. 2020;127(2):P288-P320.