This supplement summarizes a discussion on second-generation therapies and implantable devices for the treatment ofretinal diseases.
Beyond the First Generation: MAKING REAL-WORLD DECISIONS ABOUT RETINAL THERAPIES
This supplement summarizes a discussion on second-generation therapies and implantable devices for the treatment of retinal diseases.
Anti-vascular endothelial growth factor (VEGF) agents remain the standard of care for patients with neovascular age-related macular degeneration (nAMD), retinal vein occlusion (RVO), and diabetic macular edema (DME).1-3 However, the treatment burden for patients is often significant, with many requiring frequent injections.4 In addition, disease control with first-generation anti-VEGF agents may not be optimal due to clinical factors or a lack of consistent follow-up.5,6 The approval of second-generation agents with superior drying capability and greater durability offers an opportunity to improve outcomes for patients.7-10 When and how to switch to a second-generation agent is a complex decision that must be tailored to the clinical scenario. The following case presentations provide examples of how to effectively implement these agents in various real-world settings.
CASE 1
Danny A. Mammo, MD: Our first case is a 91-year-old patient with nAMD in both eyes who presented for follow-up. The patient was receiving aflibercept 2 mg every 4 to 5 weeks in the right eye and bevacizumab every 4 weeks in the left eye. On examination, VA was 20/70 in the right eye and 20/400 in the left eye. In the right eye, there was retinal pigment epithelium (RPE) mottling and atrophy. In the left eye, there was end-stage nAMD with a disciform scar and a severe submacular hemorrhage (Figure 1). The left eye had previously undergone surgical treatment for submacular hemorrhage with subretinal tissue plasminogen activator, bevacizumab, fluid-air exchange, and gas many years ago.
The patient remained stable on this regimen for several years. However, at a subsequent follow-up visit, she developed a submacular hemorrhage in the right eye (Figure 2). VA in the right eye dropped to 20/200.
The decision was made to change therapy to faricimab in the right eye. On subsequent follow-up, the submacular hemorrhage had resolved, and VA improved to 20/100 (Figure 3). Dr. Leng, what has been your experience in switching patients to newer anti-VEGF agents such as faricimab?

Figure 1. The right eye had RPE mottling, atrophy, and scars from prior laser retinopexies.The left eye had a severe submacular hemorrhage and disciform scar.

Figure 2. OCT showing PED, subretinal hyperreflective material, and subretinal fluid consistent with a new submacular hemorrhage in the right eye.

Figure 3. OCT after switching treatment to faricimab showing resolution of the submacular hemorrhage in the right eye.
Theodore Leng, MD, FACS: We are very fortunate right now to have so many options for our patients, especially those with nAMD. I have seen some benefit of switching to newer second-generation agents in eyes that are recalcitrant or requiring very frequent therapy. We have seen greater efficacy with faricimab, aflibercept 8 mg, and, in the past, brolucizumab, in these types of eyes.
Dr. Mammo: When you see a treatment-naïve patient who has a pigment epithelial detachment (PED) with surrounding subretinal fluid or subretinal hyperreflective material, how would you approach treatment?
Dr. Leng: When you see a PED, especially if it is large, there is concern about the potential for an RPE tear. Greater PED height has been shown to correlate with the risk of an RPE tear with treatment.11 But the reality is, you must treat the patient regardless, because if you do not treat them, they will lose vision due to the nAMD activity. Therefore, I initiate anti-VEGF therapy. I often counsel patients about this potential risk. However, sometimes I do not bring it up if it is not going to change our management. What has your experience been with these types of patients?
Dr. Mammo: For very large PEDs, I do worry about RPE tears, especially with the newer anti-VEGF agents, which are effective at drying up PEDs.12 However, there is not much you can do to prevent tears. I let the patient know that if I do not treat them, their vision will get worse. However, there is a small chance that if the treatment works too quickly, it might lead to more vision loss. I let them know that I would still get this treatment if it were me. Dr. Leng, when you have a monocular patient, like this case, and the patient asks if they can ever stop treatment, what do you tell them?
Dr. Leng: In a patient who has lost vision in one eye, you must protect the remaining eye as much as possible. I am very upfront and let patients know that, unfortunately, this will likely be a lifelong therapy with the current technology. I also like to be as conservative as possible with treatment. I tend not to extend the treatment interval in a monocular patient as much as I would in other patients.
Dr. Mammo: I try to keep the interval somewhere between 12 to 18 weeks in monocular patients. I typically do not extend the interval past 20 weeks, which we know is possible with faricimab and aflibercept 8 mg based on the TENAYA, LUCERNE, and PULSAR studies.7,8,13 You made a great point about overtreating rather than undertreating—I tell these patients that if it were my eye, I would want injections for life.
We are entering a very exciting time, because the DRCR Retina Network is investigating home OCT in the Protocol AO study.14 This is a multicenter, randomized trial planning to enroll approximately 600 eyes. Patients are randomized to treat-and-extend versus home OCT-guided treatment with faricimab. The primary outcomes are the mean change in visual acuity and the difference in the number of injections from baseline at 104 weeks.14 In the future, home OCT will allow us to offer more personalized treatment. However, right now, treat-and-extend is the preferred method, because it leads to better visual outcomes compared with pro re nata (PRN) treatment.15 What are your thoughts on that, Dr. Leng?
Dr. Leng: I think that home OCT is the future of our field. It is going to allow us to personalize the treatment and intervals for the patient’s benefit, reduce the number of visits to the clinic, and reduce the number of procedures.
Dr. Mammo: If a patient has a submacular hemorrhage, such as in our case presentation, does this affect which agent you choose?
Dr. Leng: I tend to select a more powerful medication if I see a submacular hemorrhage, because this indicates a higher level of disease activity.16 Therefore, I would choose a second-generation agent to clear up the hemorrhage, improve vision, and preserve photoreceptors as much as possible.
Dr. Mammo: Some data suggest that the newer anti-VEGF agents may prevent or treat submacular hemorrhages more effectively. There was a retrospective study of over 9,000 eyes that looked at anti-VEGF agents and the rates of submacular hemorrhage.17 Notably, this study did not include aflibercept 8 mg. In eyes with submacular hemorrhage, the last injection received was bevacizumab in 38%, aflibercept 2 mg in 35%, ranibizumab in 25%, and faricimab in 2%. The older agents—like aflibercept 2 mg, bevacizumab, and ranibizumab—had rates of submacular hemorrhage from 0.41% to 0.63%, while faricimab had a rate of 0.21%, which was significantly lower.17 That could be due to the angiopoietin-2 (Ang-2) effect of faricimab.
Dr. Leng: I definitely think that Ang-2 inhibition is giving us something beyond anti-VEGF. We have seen some data recently where, looking at central subfield thickness (CST) in different trials, there has been an additional benefit from Ang-2 inhibition compared with VEGF inhibition alone.7
Dr. Mammo: Unfortunately, we do not have a head-to-head comparison of the second-generation agents. However, in TENAYA and LUCERNE, which compared faricimab with aflibercept 2 mg, there was a greater absence of intraretinal and subretinal fluid in the faricimab groups in the dose-matched loading phase through week 12.7 This suggests a greater drying effect of faricimab. PULSAR, which compared aflibercept 8 mg every 12 or 16 weeks with aflibercept 2 mg every 8 weeks, had similar findings.8 The aflibercept 8 mg groups had greater proportions of patients with absence of fluid in the center subfield compared with aflibercept 2 mg in the loading phase and at year 1.8
In this case, we also discussed PED height and risk of RPE tears. In post hoc analyses of TENAYA and LUCERNE, there was a greater reduction in PED thickness for faricimab compared with aflibercept 2 mg in the dose-matched phase at weeks 4, 8, and 12.12 Faricimab also had a faster time to achieve a 50% reduction of maximum PED thickness.18 The rates of RPE tears were 2.9% and 1.5% in the faricimab and aflibercept 2 mg groups, respectively.18 It is something to be mindful of for our patients receiving these newer treatments.
CASE 2
Dr. Leng:The next case is a 61-year-old patient with intermediate dry AMD in the right eye and nAMD in the left eye. VA was 20/25 in the right eye and 20/70 in the left eye. OCT of the right eye showed a mild epiretinal membrane and large drusen (Figure 4). In the left eye, there was intraretinal fluid adjacent to a choroidal neovascular membrane.

Figure 4. (A) OCT of the right eye showed a mild epiretinal membrane and large drusen consistent with intermediate AMD. (B) In the left eye, there was intraretinal fluid adjacent to a choroidal neovascular membrane.

Figure 5. (A) Four weeks after treatment with aflibercept 8 mg, the left eye showed trace intraretinal fluid, and the patient had metamorphopsia. (B) Four weeks after treatment with faricimab, the retina was dry. (C) Five weeks after receiving faricimab, there was a return of intraretinal and subretinal fluid.
The patient was initially treated with bevacizumab, then switched to aflibercept 2 mg, then aflibercept 8 mg, all without a good anatomical or functional response. Four weeks after treatment with aflibercept 8 mg, there was a trace amount of subretinal fluid in the center of the fovea (Figure 5A). Although VA was 20/20 -3, the patient noted metamorphopsia. Most recently, the patient received a total of 40 injections of faricimab. When receiving faricimab at a 4-week interval, the retina was dry and VA was 20/20 -1 (Figure 5B). Although the patient remained stable on faricimab every 4 weeks, when the interval was extended to 5 weeks, OCT showed leakage (Figure 5C). Clearly, this is a patient who has a very high treatment burden. Dr. Mammo, have you had any patients who have been similar to this case?
Dr. Mammo: Yes, unfortunately, I have had a few patients like this who require injections every 4 or 5 weeks. These cases are humbling, because although we have aflibercept 8 mg with a higher molar dose of anti-VEGF and faricimab with the dual inhibition of VEGF and Ang-2, we still see some patients with persistent fluid. Perhaps there are other mechanisms that we are not aware of.
Dr. Leng: Have you ever tried combination treatment with anti-VEGF and photodynamic therapy in these eyes?
Dr. Mammo: I used to reserve photodynamic therapy for central serous chorioretinopathy but, more recently, I have been using it for some patients with nAMD as well, primarily to help reduce persistent fluid and to allow for extended treatment intervals.
Dr. Leng: This case is a very extreme example where the patient required monthly treatment with faricimab. However, while patients in TENAYA and LUCERNE had treatment intervals of 12 to 16 weeks and greater, why do you think we are not always seeing that consistently in real-world settings?
Dr. Mammo: That is a great question. Clinical trials have strict inclusion and exclusion criteria, but our patients do not always meet the same criteria. We see patients with a variety of health issues, lesion types, and vision ranges that might not have been included in the trials. While clinical trials primarily include treatment-naïve patients, many of us use the newer agents for patients who are treatment-resistant and have already been on other medications.7,8 In clinical trials, they are also very strict about administering loading doses, which may not be the case in the real world. That might also affect the durability of these medications. And lastly, in PULSAR, TENAYA, and LUCERNE, treatment intervals could not be shortened below 8 weeks.7,8 And we know that is not what happens in the real world.
Dr. Leng: I think the clinical trials are limited in certain ways and overoptimized in other ways. We are also not seeing patients monthly like we do in clinical trials. Do you think we are generally tolerating less fluid in the real world than in the clinical trials?
Dr. Mammo: That is another great question. In DME, we know we can tolerate some fluid, but in nAMD, that may not be the case. In clinical trials for nAMD, the retreatment criteria do allow for a small amount of fluid. Whereas in our clinics, we might see any amount of fluid and give another injection. Many of us also treat intraretinal and subretinal fluid differently. What is your approach to the different types of fluid?
Dr. Leng: There has been some data that showed that a small amount of subretinal fluid can be tolerated in nAMD if the patient’s vision is preserved and they are not symptomatic.19.20 In my practice, I do tolerate a little bit of subretinal fluid, especially if the patient is not mentioning any increase in symptoms. Dr. Mammo, have you seen patients with good visual acuity who are still symptomatic?
Dr. Mammo: There are vision parameters that are not measured by Snellen visual acuity, such as contrast sensitivity or low light luminance, that patients can notice and might not be picked up on the Snellen chart.
Dr. Leng: Do you think that a zero-order kinetic treatment, such as an implant or the port delivery system (PDS), could help to even out variances during the time between injections?
Dr. Mammo: The PDS is a newer treatment modality that allows constant anti-VEGF delivery.21 There is some suggestion that fluctuations in fluid may lead to vision loss and fibrosis.22-24 Constant anti-VEGF delivery may reduce these fluctuations in patients who are very difficult to treat and require frequent injections.25
Dr. Leng: I have had a great experience with long-duration implants, because you get a consistent low level of drug delivered. I do think that affects the pathophysiology of the conditions we are treating. Even in the TENAYA, LUCERNE, and PULSAR phase 3 pivotal trials, not all patients were able to be extended out to longer intervals.7,8,26
In addition, simply increasing the dose of anti-VEGF alone does not necessarily result in better anatomic outcomes.7 In the HARBOR study, ranibizumab was given at a quadrupled dose of 2 mg. There was no difference in the central foveal thickness (CFT) between the usual 0.5-mg dose and the 2-mg dose.27 Similarly, in PULSAR, there was no difference in central retinal thickness (CRT) between aflibercept 8 mg and 2 mg at 4, 8, and 12 weeks.8
Dr. Leng: In summary, a subset of patients with nAMD continue to have high treatment burdens, even with the second-generation anti-VEGF agents. These agents do offer better disease control. The addition of Ang-2 suppression appears to increase drying, whereas increasing anti-VEGF suppression alone may not necessarily do the same.
CASE 3
Yasha S. Modi, MD, MHS: The next case is a 70-year-old man with a history of a branch RVO (BRVO) in the right eye with macular edema since 2017 who presented for a second opinion. His initial VA was 20/100, which improved to 20/50 after treatment. He had received 22 bevacizumab injections, 4 ranibizumab injections, 18 aflibercept 2 mg injections, and 4 triamcinolone injections. He had been unable to extend beyond a 4-week interval with aflibercept and a 6-week interval with triamcinolone. I treated with the dexamethasone implant, and we were able to extend to about 10 weeks. The patient received a total of 13 injections of the dexamethasone implant over the course of several years and maintained 20/60 VA (Figure 6).
However, after the 14th dexamethasone injection, the patient presented with endophthalmitis (Figure 7). VA was hand motion, and there was a hypopyon and vitritis. OCT showed no macular edema. Unfortunately, this is an inevitable outcome for a minority of our patients. Endophthalmitis occurs in approximately 1 in 500 patients who receive the dexamethasone implant compared with about 1 in 2,000 who are treated with anti-VEGF agents.28,29 The patient underwent a vitreous tap and injection of vancomycin and ceftazidime on 2 separate visits. The culture grew Staphylococcus epidermitis, which is overall a very good prognosis for this patient. He then underwent a vitrectomy.
Three months later, VA in the right eye had improved to 20/100. There are some reports that after endophthalmitis in patients with nAMD, the treatment burden decreases; therefore, we opted to pause treatment.30 Upon follow-up 5 months after vitrectomy, the patient reported that his vision had worsened. VA was 20/200, and there was a recurrence of macular edema (Figure 8A). I treated with aflibercept 2 mg.
Four weeks later, there was persistent macular edema. I suggested switching to faricimab, but the patient preferred to remain on aflibercept 2 mg—he was hesitant to try anything new due to the history of endophthalmitis. His vision continued to worsen on aflibercept, so we switched to the dexamethasone implant due to the patient’s preference for that treatment. Unfortunately, there was no response. I then treated with off-label aflibercept 8 mg, but there was again no response (Figure 8B). The patient opted for the dexamethasone implant again, but there was still no treatment response (Figure 8C). At that point, I convinced him to switch to faricimab. Four weeks later, the macular edema had resolved, and VA improved to 20/80+ (Figure 8D). He continued with faricimab at a 4-week interval over the next several months and has maintained VA at approximately 20/80 to 20/100. The treatment interval cannot be extended beyond 4 weeks without recurrence of the macular edema.
Ferhina S. Ali, MD, MPH, FASRS: We know that there is a subset of patients who require treatment every 4 weeks. As we have seen in this patient, some also require combination therapy with corticosteroids. Tell me a little bit about your practice pattern for combination therapy.
Dr. Modi: As in this case, I often introduce corticosteroids to extend the treatment interval. I prefer corticosteroid monotherapy, but many patients do need combination therapy. This patient also had an untreatable component of macular ischemia, which was evident on OCT angiography. Therefore, this was likely the best visual outcome that we were able to achieve.
In BALATON and COMINO, the registration studies for faricimab in RVO, patients received monthly injections of faricimab or aflibercept 2 mg for 24 weeks.31 Faricimab demonstrated noninferiority to aflibercept for visual acuity gains at 24 weeks, which was the primary endpoint.32 On fluorescein angiography at 24 weeks, there was some benefit for faricimab in reducing macular leakage relative to aflibercept 2 mg.32 After 24 weeks, a treat-and-extend protocol was implemented.31 It will be interesting to see the 2-year results and the durability of this medication. Currently, there is very little evidence to support the superiority of one medication over the other. This is where real-world studies will come into play.
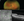
Figure 6. (A) Fundus photograph of the patient at presentation and (B) OCT after treatment with the dexamethasone implant.

Figure 7. (A) Five days after the 14th injection of the dexamethasone implant, the patient presented with endophthalmitis. (B) OCT showed no macular edema.
CASE 4
Durga Borkar, MD, MMCi: The next case is a 65-year-old woman with moderate nonproliferative diabetic retinopathy (NPDR) with persistent DME who was considering cataract surgery. On examination, VA was 20/50, and OCT demonstrated center-involved DME (Figure 9). After 3 monthly injections of bevacizumab, which were required by her insurance, VA was 20/40, and there was minimal improvement. Dr. Talcott, are you surprised by this result?

Figure 8. (A) Five months after vitrectomy, there was recurrence of macular edema. (B) Four weeks after aflibercept 8 mg, there was no improvement. (C) There continued to be no treatment response 4 weeks after the dexamethasone implant. (D) Four weeks after injection of faricimab, the macular edema had resolved.

Figure 9. (A) The patient at presentation and (B) after 3 injections of bevacizumab.

Figure 10. After 2 injections of aflibercept 2 mg, there was some improvement in the edema.
Katherine E. Talcott, MD: This patient has several findings on her initial OCT that would make me concerned that her DME would be hard to treat. These include subretinal fluid, hyperreflective foci, and very large cysts. Therefore, I am a little surprised she did as well as she did after these 3 injections.
Dr. Borkar: After the 3 bevacizumab injections, I was able to switch to aflibercept 2 mg. After 2 injections of aflibercept 2 mg every 4 weeks, her vision remained stable, and there was some improvement in the edema, particularly temporally (Figure 10). She wanted to proceed with cataract surgery. Dr. Talcott, at this point, what would you do?
Dr. Talcott: Cataract surgery can cause inflammation that can worsen DME, so ideally, DME should be controlled before cataract surgery. However, if we wait for the fluid to be completely resolved, some patients might not ever be able to have surgery. For a patient like this who has been getting regular injections, I am fine with them proceeding with cataract surgery. I tell the patient that it is important that we continue to monitor and treat them around the time of the surgery. I think you could also consider switching to either a corticosteroid or a second-generation anti-VEGF agent.
Dr. Borkar: In this case, the cataract surgeon wanted the edema to be better controlled. I did not have access to aflibercept 8 mg, so we discussed faricimab and corticosteroids. For the patient, the idea that a corticosteroid might make the cataract worse, even temporarily, was not appealing to her. So, we decided to transition to faricimab. How do you manage the transition from one agent to another?
Dr. Talcott: It depends on the situation. If it is a patient whose fluid is well controlled, I will keep them at the same interval initially after I switch and then extend them. But for a case like this, where there is recalcitrant fluid, I will continue at the same treatment interval for a longer duration until the OCT is as dry as I can get it, because that gives me a sense of what their visual potential is. Once I understand that, I might extend the interval. If the patient is undergoing cataract surgery, I would continue to treat every 4 weeks around the time of the surgery.
Dr. Borkar: I gave the patient 4 loading doses of faricimab every 4 weeks. VA improved to 20/25, and the DME was well controlled. However, there was some ellipsoid zone disruption on the OCT (Figure 11).
I wish I could have started with faricimab right up front, because maybe the OCT would have looked a little bit better. After cataract surgery and an additional 5 injections at progressively increasing intervals up to 8 weeks, the VA was 20/20 (Figure 12). With these types of patients, do you continue to treat with treat-and-extend or change to a PRN approach?
Dr. Talcott: Although I am definitely more likely to use a PRN strategy in DME as opposed to nAMD, because it took a long time to get to this point with regular injections, I would begin with a treat-and-extend approach. Once I achieved extended intervals, then I would consider switching to PRN. The other thing that you will have to keep a watch for as you extend is proliferative diabetic retinopathy (PDR).
Dr. Borkar: I also prefer a treat-and-extend approach in these situations. Let's review the data behind some of these newer agents. YOSEMITE and RHINE were the phase 3 registration studies looking at faricimab for DME.9 On average, patients gained about 10 letters from baseline and had an approximately 200 µm CST reduction that they were able to maintain at the end of 2 years.9 RHONE-X was a multicenter extension study looking at patients who completed YOSEMITE and RHINE in years 3 and 4.33 The aflibercept 2-mg arm crossed over to receive faricimab during the open-label period after week 116. More than 90% of patients achieved absence of DME by the end of RHONE-X, regardless of which treatment arm they started in.33 The BCVA and CST improvements were also maintained.33,34 Almost 80% of patients had at least a 12-week treatment interval at the end of 4 years.33 The safety signals were very similar to those in YOSEMITE and RHINE.33
FARETINA-DME was a retrospective, real-world study using data from the Intelligent Research in Sight (IRIS) Registry.35 Approximately 90% of eyes were previously treated, and about 75% had switched from aflibercept 2 mg.35 Notably, more than 50% of patients in this study had 20/40 or better VA at baseline, which would not qualify for a clinical trial.35 At 2 years, visual acuity improved in treatment-naïve eyes, as we would expect, but in previously treated eyes, visual acuity was maintained. That might not sound very impressive, but in most real-world studies, we usually see vision declining over time, especially in previously treated eyes. There were CST improvements through 2 years of faricimab treatment, with a decrease in the number of injections.35 In the first 6 months, both treatment-naïve and previously treated patients received about 4 injections, but this declined over time.35 The safety profile was very similar to what we saw in YOSEMITE and RHINE in terms of endophthalmitis and intraocular inflammation.35

Figure 11. After 4 loading doses of faricimab, the macular edema had resolved, but there was ellipsoid zone disruption.

Figure 12. After cataract surgery and 5 injections of faricimab up to every 8 weeks, the macula remained dry.
CASE 5
Dr. Talcott: The next case is a 64-year-old man with moderate NPDR in the right eye. He was monocular with a history of left eye enucleation due to an injury in childhood. He had received multiple injections of aflibercept 2 mg for DME over 5 years. Unfortunately, throughout his treatment, he had many unintended lapses in ophthalmic care. He presented for follow-up 6 weeks after his last aflibercept injection. VA was 20/30, and there was significant center-involved DME (Figure 13).
He was supposed to return in 4 to 6 weeks, but unfortunately, he returned 9 months later. VA was 20/40, and OCT showed an increase in intraretinal fluid (Figure 14).
He was restarted on aflibercept 2 mg. Four weeks later, VA was 20/30, and there was persistent fluid on the OCT, though it was slightly improved. I wanted him to return 4 weeks later but, unfortunately, he came back 8 weeks later. Vision was stable, and the OCT was unchanged or slightly worse. Dr. Borkar, what are your thoughts on this case?
Dr. Borkar: This is not dissimilar to many patients that I see in my practice with multiple unintended treatment lapses. Corticosteroids can be a great idea for patients who have a large central cyst on OCT. However, this patient’s monocular status and the risks of elevated IOP and cataract make me more hesitant to consider that.1 So I might consider aflibercept 8 mg or faricimab as a next step rather than corticosteroids in this case.
Dr. Talcott: I switched the patient to aflibercept 8 mg at this point. Four weeks later, VA was unchanged at 20/30, but the intraretinal fluid was a little bit better. What is your process like for switching patients to second-generation anti-VEGF agents?
Dr. Borkar: It depends. If the disease is well controlled and I am switching the patient primarily for durability, I may not use loading doses. However, in this case, the disease was not well controlled, and I would definitely load. At the end of the loading phase, I would love to see that the central intraretinal cysts are much better controlled. If so, we might have an opportunity to extend the interval. However, the amount we would be able to extend is difficult to predict.
Dr. Talcott: Most of the patients in the clinical trials for aflibercept 8mg and faricimab were treatment-naïve. Therefore, it is difficult to apply those protocols to patients we are treating for recalcitrant fluid. Unfortunately, although I wanted the patient to come back in 4 weeks, they returned at 12 weeks. With continued treatment at inconsistent follow-up intervals over the following months, VA was 20/20 after a total of 4 injections of aflibercept 8 mg. Dr. Borkar, do you think we should consider agents with improved drying and durability earlier in monocular patients or those who have been lost to follow-up?
Dr. Borkar: Improved drying and durability earlier in the treatment course are important. There are data to suggest that once patients have had persistent fluid and exudates, even once the macular edema is controlled, visual acuity may not recover to baseline.36 However, when patients are lost to follow-up, it is hard to assess treatment responses. The PDS, in theory, could be a great idea for this patient. Although we are focused on DME here, peripheral retinopathy can progress without consistent treatment. The PAGODA study for the PDS demonstrated the ability to control peripheral retinopathy.37 However, this patient’s monocular status and inconsistent follow-up history might make me a little bit nervous about an implant for him.

Figure 13. OCT at presentation showed center-involved DME.

Figure 14. OCT of the same patient after a 9-month lapse in care.
Dr. Talcott: With lapses in care, you worry that if there is a complication, such as exposure of the implant, you may not catch it at an early stage where you can still intervene. This case highlights that patients with diabetic retinopathy can often have multiple barriers to care. A retrospective cohort study of patients with NPDR and DME found that 25% of patients had loss to follow-up (LTFU), defined as a 12-month or greater gap between office visits following an injection.6 Risk factors included race or ethnicity and adjusted gross income. In another retrospective study, patients who were LTFU for at least 6 months after an injection had an initial visual acuity decline.38 However, there was no significant change in visual acuity from baseline at 3, 6, or 12 months or at the final follow-up. This suggests that there might be opportunities to recover vision after LTFU. In a post hoc analysis of patients from the DRCR Protocol S who were treated with ranibizumab for PDR, over 55% of patients had 1 or more lapses in care of at least 8 weeks.39 This was despite having a research coordinator who was frequently checking in with patients, which makes it less likely to have a lapse in care.
Medications with greater durability might be able to help with this. In the PHOTON phase 3 clinical trial, patients who received aflibercept 8 mg every 12 or 16 weeks had noninferior visual acuity gains despite receiving fewer injections.10 In the PHOTON extension study, patients were followed to year 3. Patients on aflibercept 2 mg every 8 weeks were switched to aflibercept 8 mg every 12 weeks, and those on aflibercept 8 mg continued at the last assigned dosing interval.40 Patients maintained visual acuity gains and CRT from the PHOTON study. Almost 50% of patients were extended to an interval of 20 weeks.40
Early real-world studies have also looked at how retina specialists integrated aflibercept 8 mg into their practices. A real-world study of patients with DME used data from the IRIS registry and the Vestrum database.41 In the initial loading phase, treatment-naïve patients had an average of 41 and 42 days between injections for IRIS and Vestrum, respectively. After the loading phase, the mean intervals were 77 and 75 days.41 Most previously treated patients in this study were switched from aflibercept 2 mg to 8 mg.41 In those who had a baseline injection interval of every 4 to 6 weeks, there was an average extension by about 3 to 4 weeks.41 In those with a baseline interval of 6 to 8 weeks, there was an average extension of 2 to 3 weeks.41
In summary, as we know, lapses in the care of patients with DME are not uncommon. Agents with improved drying and durability may be able to reduce the treatment burden for these patients. However, this must be balanced with the need for appropriate monitoring of patients and insurance approval of these agents.
1. Lim JI, Kim SJ, Bailey ST, et al. Diabetic retinopathy Preferred Practice Pattern®. Ophthalmology. 2025;132(4):75-162.
2. Vemulakonda GA, Bailey ST, Kim SJ, et al. Age-related macular degeneration Preferred Practice Pattern®. Ophthalmology. 2025;132(4):1-74.
3. Kovach JL, Bailey ST, Kim SJ, et al. Retinal vein occlusions Preferred Practice Pattern®. Ophthalmology. 2025;132(4):303-343.
4. Wang R, McClard CK, Laswell S, et al. Quantifying burden of intravitreal injections: questionnaire assessment of life impact of treatment by intravitreal injections (QUALITII). BMJ Open Ophthalmol. 2022;7(1):e001188.
5. Ciulla TA, Kapik B, Hu A, Harris A, Ip MS, Blodi B. Anatomic biomarkers of macular edema associated with retinal vein occlusion. Ophthalmol Retina. 2022;6(12):1206-1220.
6. Gao X, Obeid A, Adam MK, Hyman L, Ho AC, Hsu J. Loss to follow-up in patients with retinal vein occlusion undergoing intravitreal anti-VEGF injections. Ophthalmic Surg Lasers Imaging Retina. 2019;50(3):159-166.
7. Khanani AM, Kotecha A, Chang A, et al. TENAYA and LUCERNE: two-year results from the phase 3 neovascular age-related macular degeneration trials of faricimab with treat-and-extend dosing in year 2. Ophthalmology. 2024;131(8):914-926.
8. Lanzetta P, Korobelnik JF, Heier JS, et al. Intravitreal aflibercept 8 mg in neovascular age-related macular degeneration (PULSAR): 48-week results from a randomised, double-masked, non-inferiority, phase 3 trial. Lancet. 2024;403(10432):1141-1152.
9. Wong TY, Haskova Z, Asik K, et al. Faricimab treat-and-extend for diabetic macular edema: two-year results from the randomized phase 3 YOSEMITE and RHINE trials. Ophthalmology. 2024;131(6):708-723.
10. Brown DM, Boyer DS, Do DV, et al. Intravitreal aflibercept 8 mg in diabetic macular oedema (PHOTON): 48-week results from a randomised, double-masked, non-inferiority, phase 2/3 trial. Lancet. 2024;403(10432):1153-1163.
11. Yasuhara S, Miyata M, Ooto S, et al. Predictors of retinal pigment epithelium tear development after treatment for neovascular age-related macular degeneration using swept source optical coherence tomography angiography. Retina. 2022;42(6):1020-1027.
12. Avery RL, Kotecha A, et al. Key clinical and anatomical outcomes with faricimab in patients with nAMD: results from the TENAYA/LUCERNE trials. Presented at: American Society of Retina Specialists 2024 Annual Meeting; July 17-20, 2024; Stockholm, Sweden.
13. Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729-740.
14. Home OCT-guided treatment versus treat and extend for the management of neovascular AMD. DRCR Retina Network. Accessed June 28, 2025. https://public.jaeb.org/drcrnet/stdy/597
15. Rosenberg D, Deonarain DM, Gould J, et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: a systematic review and meta-analysis. Eye (Lond). 2023;37(1):6-16.
16. Gabrielle PH, Maitrias S, Nguyen V, et al. Incidence, risk factors and outcomes of submacular haemorrhage with loss of vision in neovascular age-related macular degeneration in daily clinical practice: data from the FRB! registry. Acta Ophthalmol. 2022;100(8):e1569-e1578.
17. Kaufmann GT, Boucher N, Sharma C, Aggarwal N, Starr MR. Submacular hemorrhage rates following anti-vascular endothelial growth factor injections for exudative age-related macular degeneration. Am J Ophthalmol. 2025;270:172-182.
18. Avery RL, Lai TY, Souverain A, et al. Greater reduction in pigment epithelial detachment size with faricimab vs aflibercept during head-to-head dosing in patients with nAMD. Presented at: Macula Society 47th Annual Meeting; February 7-10, 2024; Palm Springs, CA.
19. Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126(5):723-734.
20. Ohji M, Okada AA, Sasaki K, Moon SC, Machewitz T, Takahashi K. Relationship between retinal fluid and visual acuity in patients with exudative age-related macular degeneration treated with intravitreal aflibercept using a treat-and-extend regimen: subgroup and post-hoc analyses from the ALTAIR study. Graefes Arch Clin Exp Ophthalmol. 2021;259(12):3637-3647.
21. Regillo C, Berger B, Brooks L, et al. ARCHWAY phase 3 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration 2-year results. Ophthalmology. 2023;130(7):735-747.
22. Chakravarthy U, Havilio M, Syntosi A, et al. Impact of macular fluid volume fluctuations on visual acuity during anti-VEGF therapy in eyes with nAMD. Eye (Lond). 2021;35(11):2983-2990.
23. Ehlers JP, Lunasco LM, Yordi S, et al. Compartmental exudative dynamics in neovascular age-related macular degeneration: volumetric outcomes and impact of volatility in a phase III clinical trial. Ophthalmol Retina. 2024;8(8):765-777.
24. Evans RN, Reeves BC, Maguire MG, et al. Associations of variation in retinal thickness with visual acuity and anatomic outcomes in eyes with neovascular age-related macular degeneration lesions treated with anti-vascular endothelial growth factor agents. JAMA Ophthalmol. 2020;138(10):1043-1051.
25. Sheth VS, Holekamp NM, Khanani AM, et al. Retinal fluid and thickness fluctuations in Archway trial for port delivery system with ranibizumab versus monthly ranibizumab injections. Ophthalmol Retina. 2025;9(4):330-342.
26. Brown D, et al. Efficacy and safety of high-dose (8.0 mg) aflibercept for treatment for wet AMD: 48-Week results from PULSAR. Presented virtually at: Angiogenesis, Exudation, and Degeneration; February 10-11, 2023.
27. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-1056.
28. Stem MS, Todorich B, Yonekawa Y, Capone A Jr, Williams GA, Ruby AJ. Incidence and visual outcomes of culture-proven endophthalmitis following dexamethasone intravitreal implant. JAMA Ophthalmol. 2017;135(4):379-382.
29. McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31(4):654-661.
30. Kokame GT, Yannuzzi NA, Shantha JG, et al. Involution of neovascular age-related macular degeneration after endophthalmitis. Retin Cases Brief Rep. 2021;15(5):495-499.
31. Hattenbach LO, Abreu F, Arrisi P, et al. BALATON and COMINO: phase III randomized clinical trials of faricimab for retinal vein occlusion: study design and rationale. Ophthalmol Sci. 2023;3(3):100302.
32. Tadayoni R, Paris LP, Danzig CJ, et al. Efficacy and safety of faricimab for macular edema due to retinal vein occlusion: 24-week results from the BALATON and COMINO trials. Ophthalmology. 2024;131(8):950-960.
33. Khanani AM, Kotecha A, Harrell E, et al. Four-year outcomes of faricimab in DME: first time safety and efficacy results from the RHONE-X long-term extension trial. Presented at: American Society of Retina Specialists 2024; July 17-20, 2024; Stockholm, Sweden.
34. Khanani AM, Kotecha A, Margaron P, et al. Faricimab in nAMD and DME: latest updates. Presented at: Angiogenesis, Exudation, and Degeneration; February 10-11, 2023.
35. Singh R. Interpreting the latest real-world data on nAMD. Presented at: Association for Research in Vision and Ophthalmology Annual Meeting 2025; May 4-8, 2025; Salt Lake City, UT.
36. Santos AR, Costa M, Schwartz C, et al. Optical coherence tomography baseline predictors for initial best-corrected visual acuity response to intravitreal anti-vascular endothelial growth factor treatment in eyes with diabetic macular edema: the CHARTRES study. Retina. 2018;38(6):1110-1119.
37. Wirthlin R, Gill M, Howard D, Menezes A, Rahman S, Latkany P. Port delivery system with ranibizumab (PDS) stabilizes retinal nonperfusion and macular leakage: results from Pagoda and Pavilion phase 3 trials in diabetic macular edema (DME) and diabetic retinopathy (DR). Invest Ophthalmol Vis Sci. 2024;65(7):6232.
38. Matsunaga DR, Salabati M, Obeid A, et al. Outcomes of eyes with diabetic macular edema that are lost to follow-up after anti–vascular endothelial growth factor therapy. Am J Ophthalmol. 2022;233:1-7.
39. Maguire MG, Liu D, Bressler SB, et al. Lapses in care among patients assigned to ranibizumab for proliferative diabetic retinopathy: a post hoc analysis of a randomized clinical trial. JAMA Ophthalmol. 2021;139(12):1266-1273.
40. Do DV. Aflibercept 8 mg in DME: key results from the PHOTON extension study. Presented at: American Academy of Ophthalmology 2024 Annual Meeting; October 18-21, 2024; Chicago, IL.
41. Mehta N, et al. Early insights on the real-world use of aflibercept 8 mg among treatment-naive eyes with diabetic macular edema. IOVS. 2025;66:2398.
Target Audience
This certified continuing education (CME) activity is designed for ophthalmologists involved in the management of patients with retinal diseases.
Grantor Statement
This activity is supported by an independent educational grant from Genentech, a member of the Roche Group.
Learning Objectives
Upon completion of this activity, the participant should be able to:
- Outline the different factors that may guide the integration of second-generation therapies into treatment protocols for retinal diseases
- Infer the potential efficacy and durability of second-generation agents, based on clinical trials and real-world studies
- Discuss the clinical indicators and other nonclinical considerations involved in tailoring treatment with second-generation agents
Accreditation and Credit Designation Statements
Provided by Evolve Medical Education
Evolve is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Evolve designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Faculty
Katherine E. Talcott, MD
Program Chair
Assistant Professor of Ophthalmology
Cleveland Clinic Lerner College of Medicine
Cole Eye Institute
Cleveland Clinic
Cleveland, OHFerhina S. Ali, MD, MPH, FASRS
Assistant Professor of Ophthalmology
New York Medical College
Westchester Medical Center
Valhalla, NYDurga Borkar, MD, MMCi
Associate Professor of Ophthalmology
Duke University Eye Center
Durham, NCTheodore Leng, MD, MS
Byers Eye Institute at Stanford
Stanford University School of Medicine
Palo Alto, CADanny A. Mammo, MD
Vitreoretinal Surgery & Uveitis
Cole Eye Institute, Cleveland Clinic
Assistant Professor of Ophthalmology
Cleveland Clinic Lerner College of Medicine &
Case Western Reserve University
Cleveland, OHYasha S. Modi, MD, MHS
Associate Professor of Ophthalmology
Department of Ophthalmology
Director of Tele-Retina
NYU Langone Health
New York, NYDisclosure of Relevant Financial Relationships
DISCLOSURE POLICY
In accordance with the ACCME Standards for Integrity and Independence, it is the policy of Evolve that faculty and other individuals who are in the position to control the content of this activity disclose any real or apparent financial relationships relating to the topics of this educational activity. Evolve has full policies in place that have identified and mitigated financial relationships and conflicts of interest to ensure independence, objectivity, balance, and scientific accuracy prior to this educational activity.The following faculty/staff members have reported financial relationships with ineligible companies within the last 24 months.
Ferhina S. Ali, MD, MPH, FASRS, has had a financial relationship or affiliation with the following ineligible companies in the form of Consultant: 4DMT, AbbVie, Adverum, Apellis Pharmaceuticals, Inc., Astellas Pharma Global Development, Inc., Eyepoint Pharmaceuticals, Genentech Inc., Ocuphire, Ocuterra , Optomed, Outlook Therapeutics. Grant/Research Support: Apellis Pharmaceuticals, Inc., Astellas Pharma Global Development.
Durga Borkar, MD, MMCi, has had a financial relationship or affiliation with the following ineligible companies in the form of Consultant: Alimera Sciences, Apellis Pharmaceuticals, Astellas, EyePoint Pharmaceuticals, Genentech, Glaukos, ONL Therapeutics, and Regeneron.
Theodore Leng, MD, MS, has had a financial relationship or affiliation with the following ineligible companies in the form of Consultant: Alcon, Astellas, Boehringer Ingelheim, Genentech/Roche, Iridex, Nanoscope Therapeutics, Regeneron, Toku, Topcon, and Virtual Field. Grant/Research Support: Astellas and Luxa Biotechnology.
Danny A. Mammo, MD, has had a financial relationship or affiliation with the following ineligible companies in the form of Consultant: Alimera Sciences and Bausch + Lomb. Speaker’s Bureau: Alimera Sciences, Apellis Pharmaceuticals, and Bausch + Lomb.
Yasha S. Modi, MD, MHS, has had a financial relationship or affiliation with the following ineligible companies in the form of Consultant: AbbVie/Allergan, Alcon, Apellis Pharmaceuticals, Astellas, EyePoint Pharmaceuticals, Genentech, Notal Vision, Opthea, Regeneron, Topcon. Grant/Research Support: Genentech.
Katherine E. Talcott, MD, has had a financial relationship or affiliation with the following ineligible companies in the form of Consultant: Alimera Sciences, AbbVie, Apellis Pharmaceuticals, Bausch + Lomb, Carl Zeiss Meditec, EyePoint Pharmaceuticals, Genentech, Outlook Therapeutics, and Regeneron. Grant/Research Support: Carl Zeiss Meditec and Regeneron. Speaker’s Bureau: Apellis Pharmaceuticals and Carl Zeiss Meditec.
The Evolve, planners, reviewer, and writers have no financial relationships with ineligible companies.
Disclaimer
OFF-LABEL STATEMENT
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The opinions expressed in the educational activity are those of the faculty. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.DISCLAIMER
The views and opinions expressed in this educational activity are those of the faculty and do not necessarily represent the views of Evolve, Retina Today, or Genentech.This activity is designed for educational purposes. Participants have a responsibility to utilize this information to enhance their professional development to improve patient outcomes. Conclusions drawn by the participants should be derived from careful consideration of all available scientific information. The participant should use his/her clinical judgment, knowledge, experience, and diagnostic decision-making before applying any information, whether provided here or by others, for any professional use.
System Requirements
- Supported Browsers (2 most recent versions):
- Google Chrome for Windows, Mac OS, iOS, and Android
- Apple Safari for Mac OS and iOS
- Mozilla Firefox for Windows, Mac OS, iOS, and Android
- Microsoft Edge for Windows
- Recommended Internet Speed: 5Mbps+
Publication Dates
Expiration Date:
Ready to Claim Your Credits?
You have attempts to pass this post-test. Take your time and review carefully before submitting.
Good luck!





